Highlights
-
Full Story
Gates Institute Delivers CAR T-Cell Therapy to 50th Patient in CU Anschutz Clinical Trial
 8/7/2025
In July, the Gates Institute provided chimeric antigen receptor (CAR) T-cell therapy to the 50th patient enrolled in an investigator-led clinical trial at University of Colorado Anschutz. A pediatric patient received this individualized treatment at Children’s Hospital Colorado – in a phase 1 study open to patients with B-cell acute lymphoblastic leukemia (B-ALL) who have relapsed or whose disease didn’t respond following standard of care with chemotherapy or bone marrow transplants.
8/7/2025
In July, the Gates Institute provided chimeric antigen receptor (CAR) T-cell therapy to the 50th patient enrolled in an investigator-led clinical trial at University of Colorado Anschutz. A pediatric patient received this individualized treatment at Children’s Hospital Colorado – in a phase 1 study open to patients with B-cell acute lymphoblastic leukemia (B-ALL) who have relapsed or whose disease didn’t respond following standard of care with chemotherapy or bone marrow transplants.
-
Full Story
Gates Institute Names Michael Swabowski as Director of Operations for Gates Biomanufacturing Facility
 7/29/2025
The Gates Institute has appointed Michael Swabowski as its new director of operations for the Gates Biomanufacturing Facility (GBF). Swabowski brings more than two decades of experience leading quality and manufacturing operations across the biologics, gene therapy, and pharmaceutical industries.
7/29/2025
The Gates Institute has appointed Michael Swabowski as its new director of operations for the Gates Biomanufacturing Facility (GBF). Swabowski brings more than two decades of experience leading quality and manufacturing operations across the biologics, gene therapy, and pharmaceutical industries.
-
Full Story
Gates Biomanufacturing Facility Marks a Decade of Discovery and Innovation
 5/8/2025
When the Gates Biomanufacturing Facility opened its doors in 2015, cell and gene therapy (CGT) was just beginning to come into its own. Chimeric antigen receptor (CAR) T-cell therapies were in human clinical trials at the National Cancer Institute and messenger RNA, or mRNA, technology was quickly advancing to deliver new vaccines and treatments. Thought leaders in Colorado were keen to advance CGT research at University of Colorado Anschutz, and knew that having biomanufacturing capabilities using FDA-compliant current Good Manufacturing Practice (cGMP) regulations would be key. CGMP establishes systems for the proper design, monitoring, and
5/8/2025
When the Gates Biomanufacturing Facility opened its doors in 2015, cell and gene therapy (CGT) was just beginning to come into its own. Chimeric antigen receptor (CAR) T-cell therapies were in human clinical trials at the National Cancer Institute and messenger RNA, or mRNA, technology was quickly advancing to deliver new vaccines and treatments. Thought leaders in Colorado were keen to advance CGT research at University of Colorado Anschutz, and knew that having biomanufacturing capabilities using FDA-compliant current Good Manufacturing Practice (cGMP) regulations would be key. CGMP establishes systems for the proper design, monitoring, and
-
Full Story
Gates Institute Awards $1.5 Million to Campus Researchers from Gates Grubstake Fund
 4/22/2025
The prestigious Gates Grubstake Award is designed to accelerate the most promising projects with strong potential for clinical impact and commercial translation. Now, in its 11th year, the Grubstake Fund continues to support transformative science, helping CU Anschutz investigators overcome critical translational gaps and move their discoveries towards patient impact.
4/22/2025
The prestigious Gates Grubstake Award is designed to accelerate the most promising projects with strong potential for clinical impact and commercial translation. Now, in its 11th year, the Grubstake Fund continues to support transformative science, helping CU Anschutz investigators overcome critical translational gaps and move their discoveries towards patient impact.
-
Full Story
Bridging Bench to Bedside: Gates Institute’s Translational Sciences Lab Opens its Doors
 4/8/2025
In the spring of 2025, the Gates Institute at the University of Colorado Anschutz Medical Campus will inaugurate its Translational Sciences Lab, a pivotal initiative aimed at bridging the gap between research and clinical application in cell and gene therapy. This lab will streamline the progression of innovative therapies from the research bench to the biomanufacturing stage, expediting their journey toward clinical trials.
4/8/2025
In the spring of 2025, the Gates Institute at the University of Colorado Anschutz Medical Campus will inaugurate its Translational Sciences Lab, a pivotal initiative aimed at bridging the gap between research and clinical application in cell and gene therapy. This lab will streamline the progression of innovative therapies from the research bench to the biomanufacturing stage, expediting their journey toward clinical trials.
-
Full Story
Alzheimer’s Vaccine Manufactured at GBF Heads to Clinical Trials
 3/13/2025
Prevention of Alzheimer’s disease presents one of the most confounding challenges in modern medicine. The disease is thought to be caused by a combination of factors, the two main mechanisms being aberrantly formed beta-amyloid proteins aggregating into harmful plaques and tau proteins becoming hyperphosphorylated, leading to oxidative stress and neuronal damage.
3/13/2025
Prevention of Alzheimer’s disease presents one of the most confounding challenges in modern medicine. The disease is thought to be caused by a combination of factors, the two main mechanisms being aberrantly formed beta-amyloid proteins aggregating into harmful plaques and tau proteins becoming hyperphosphorylated, leading to oxidative stress and neuronal damage.
-
Full Story
New CAR-T Cell Therapy Shows Promise for Hard-to-Treat Cancers
 3/10/2025
Researchers at the University of Colorado Anschutz Medical Campus have successfully developed a supercharged iteration of CAR-T cell therapy that can enhance the effectiveness and longevity of the cells, particularly against cancer cells that are harder for prior CAR-T therapies to detect and fight.
3/10/2025
Researchers at the University of Colorado Anschutz Medical Campus have successfully developed a supercharged iteration of CAR-T cell therapy that can enhance the effectiveness and longevity of the cells, particularly against cancer cells that are harder for prior CAR-T therapies to detect and fight.
-
Full Story
Do Stem Cell ‘Memories’ Trigger Autoimmune Disease Flare-Ups?
 3/7/2025
Are the stem cells that form our blood and immune systems dictating the course of autoimmune disease? New research raises the possibility, suggesting that memories held within blood stem cells may trigger the often-disabling symptoms of autoimmune disease that flare up over a person’s lifetime.
3/7/2025
Are the stem cells that form our blood and immune systems dictating the course of autoimmune disease? New research raises the possibility, suggesting that memories held within blood stem cells may trigger the often-disabling symptoms of autoimmune disease that flare up over a person’s lifetime.
-
Full Story
Sujatha Venkataraman, PhD, Receives Funding for Pediatric Brain Cancer Research
 1/30/2025
The fight against diffuse intrinsic pontine glioma (DIPG), a universally fatal pediatric brain tumor, has taken a significant step forward with the award of a Team Jack Foundation grant totaling $575,000 to support groundbreaking research at the University of Colorado Anschutz Medical Campus led by Gates Institute member researcher Sujatha Venkataraman, PhD. Venkataraman is a 2023 Gates Grubstake Fund awardee and associate research professor of pediatric hematology/oncology and bone marrow transplantation in the CU School of Medicine, as well as a CU Cancer Center member. This project, “To facilitate the translation of experimental 'gated'
1/30/2025
The fight against diffuse intrinsic pontine glioma (DIPG), a universally fatal pediatric brain tumor, has taken a significant step forward with the award of a Team Jack Foundation grant totaling $575,000 to support groundbreaking research at the University of Colorado Anschutz Medical Campus led by Gates Institute member researcher Sujatha Venkataraman, PhD. Venkataraman is a 2023 Gates Grubstake Fund awardee and associate research professor of pediatric hematology/oncology and bone marrow transplantation in the CU School of Medicine, as well as a CU Cancer Center member. This project, “To facilitate the translation of experimental 'gated'
-
Full Story
CAR-T Cells Hold Memories of Past Encounters
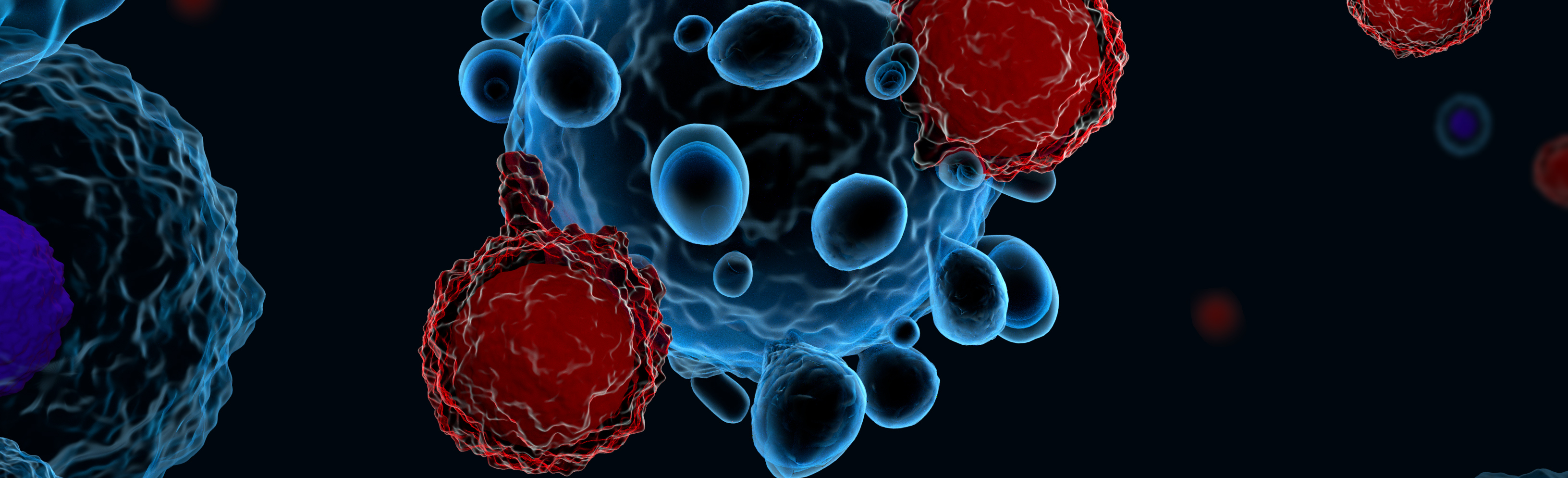 1/2/2025
Researchers at the University of Colorado Anschutz Medical Campus have discovered that some CAR-T cells engineered to fight cancer and other conditions carry the memory of past encounters with bacteria, viruses and other antigens within them, a finding that may allow scientists to manufacture the cells in more precise and targeted ways.
1/2/2025
Researchers at the University of Colorado Anschutz Medical Campus have discovered that some CAR-T cells engineered to fight cancer and other conditions carry the memory of past encounters with bacteria, viruses and other antigens within them, a finding that may allow scientists to manufacture the cells in more precise and targeted ways.
-
Full Story
Gates Institute’s Top Stories of 2024
 12/20/2024
The Gates Institute at the University of Colorado Anschutz Medical Campus had another remarkable year in 2024, marked by groundbreaking research, innovative collaborations, and the inspiring work of our community. Here are the top 10 blog posts from the past year, showcasing the highlights of our achievements and impact:
12/20/2024
The Gates Institute at the University of Colorado Anschutz Medical Campus had another remarkable year in 2024, marked by groundbreaking research, innovative collaborations, and the inspiring work of our community. Here are the top 10 blog posts from the past year, showcasing the highlights of our achievements and impact:
-
Full Story
From Patients to Investigators: Gates Summer Internship Alumni Appear on Podcast to Raise Awareness of Ehlers-Danlos Research
 12/9/2024
When Jeevan Mann, now a professional research assistant at University of Colorado Anschutz Medical Campus, was diagnosed with an ultra-rare form of the Ehlers-Danlos Syndromes (EDS) in his late teens, he immediately tried to learn more about his connective tissue disorder. There weren’t many resources for EDS patients (or for clinical providers and scientists) at that point, but one expert whose counsel he came to value was Linda Bluestein, MD, who hosts The Bendy Bodies Podcast. The program explores the science and clinical management of symptomatic joint hypermobility in a range of disorders, including
12/9/2024
When Jeevan Mann, now a professional research assistant at University of Colorado Anschutz Medical Campus, was diagnosed with an ultra-rare form of the Ehlers-Danlos Syndromes (EDS) in his late teens, he immediately tried to learn more about his connective tissue disorder. There weren’t many resources for EDS patients (or for clinical providers and scientists) at that point, but one expert whose counsel he came to value was Linda Bluestein, MD, who hosts The Bendy Bodies Podcast. The program explores the science and clinical management of symptomatic joint hypermobility in a range of disorders, including
-
Full Story
Early-career Investigator Aims to Transform Leukemia Treatment with a Novel Approach to CAR T-cell Therapy
 12/2/2024
Mathew Angelos, MD, PhD, a new assistant professor of hematology at the University of Colorado School of Medicine, is part of a wave of promising early-career physician-investigators. With a focus on bringing cutting-edge treatments from the lab to the clinic, Angelos is exploring the potential of CAR T-cell therapy to revolutionize the treatment of acute myeloid leukemia (AML) and other hematologic cancers. His ongoing research using CD64 as a target in CAR T-cell therapy is a novel and promising approach that stands to make a meaningful impact in leukemia treatment. In a recent interview,
12/2/2024
Mathew Angelos, MD, PhD, a new assistant professor of hematology at the University of Colorado School of Medicine, is part of a wave of promising early-career physician-investigators. With a focus on bringing cutting-edge treatments from the lab to the clinic, Angelos is exploring the potential of CAR T-cell therapy to revolutionize the treatment of acute myeloid leukemia (AML) and other hematologic cancers. His ongoing research using CD64 as a target in CAR T-cell therapy is a novel and promising approach that stands to make a meaningful impact in leukemia treatment. In a recent interview,
-
Full Story
New CAR T-Cell Trial Launched Through Gates Institute Packages Two CARs in One Treatment
 11/7/2024
A new phase 1 study of a chimeric antigen receptor (CAR) T-cell therapy at Children’s Hospital Colorado for pediatric patients with relapsed or refractory pre B-cell acute lymphoblastic leukemia (B-ALL) recently enrolled its first patient. The trial is the latest launched through Gates Institute, and is the fifth for which the Gates Biomanufacturing Facility produces the CAR T-cell product for research at the University of Colorado Anschutz Medical Campus.
11/7/2024
A new phase 1 study of a chimeric antigen receptor (CAR) T-cell therapy at Children’s Hospital Colorado for pediatric patients with relapsed or refractory pre B-cell acute lymphoblastic leukemia (B-ALL) recently enrolled its first patient. The trial is the latest launched through Gates Institute, and is the fifth for which the Gates Biomanufacturing Facility produces the CAR T-cell product for research at the University of Colorado Anschutz Medical Campus.
-
Full Story
In Advisory Role, Former Gates Center Associate Director Brings Decades of Expertise to Help Ready iPSC Research Program for Clinical Trials
 10/17/2024
Not long after the technology to produce induced pluripotent stem cells (iPSCs) from adult cells was developed in 2007, Mark Petrash, PhD, then-professor and vice chair of research in the Department of Ophthalmology at the University of Colorado Anschutz School of Medicine, recognized how this breakthrough would impact ophthalmology research and innovation. After joining CU Anschutz in 2008, Petrash became involved with the recently formed Gates Center for Regenerative Medicine and advocated for the goal to find a stem cell-based cure for age-related macular degeneration (AMD). The Gates Center (forerunner of Gates Institute) was
10/17/2024
Not long after the technology to produce induced pluripotent stem cells (iPSCs) from adult cells was developed in 2007, Mark Petrash, PhD, then-professor and vice chair of research in the Department of Ophthalmology at the University of Colorado Anschutz School of Medicine, recognized how this breakthrough would impact ophthalmology research and innovation. After joining CU Anschutz in 2008, Petrash became involved with the recently formed Gates Center for Regenerative Medicine and advocated for the goal to find a stem cell-based cure for age-related macular degeneration (AMD). The Gates Center (forerunner of Gates Institute) was
-
Full Story
Investigators Credit Gates Grubstake Awards with Positioning Osteoarthritis Research for Major Funding
 8/21/2024
When Gates Institute and CU Anschutz Department of Orthopedics faculty members Karin Payne, PhD, associate professor, and Mike Zuscik, PhD, professor and Orthopedics Research Vice Chair, were notified that their team’s research to develop therapies to reverse osteoarthritis had been awarded major funding from the Advanced Research Projects Agency for Health (ARPA-H), they reflected on the years of collaborative work that had led them and their colleagues to this point.
8/21/2024
When Gates Institute and CU Anschutz Department of Orthopedics faculty members Karin Payne, PhD, associate professor, and Mike Zuscik, PhD, professor and Orthopedics Research Vice Chair, were notified that their team’s research to develop therapies to reverse osteoarthritis had been awarded major funding from the Advanced Research Projects Agency for Health (ARPA-H), they reflected on the years of collaborative work that had led them and their colleagues to this point.
-
Full Story
Major Project to Address ‘Urgent Need’ For Improved Testing of Cancer Therapies
 8/21/2024
A multiyear project on the University of Colorado Anschutz Medical Campus to improve models for evaluating potential cancer-fighting therapies before they go to clinical trials has been awarded the first of what researchers hope will be several grants from the National Cancer Institute.
8/21/2024
A multiyear project on the University of Colorado Anschutz Medical Campus to improve models for evaluating potential cancer-fighting therapies before they go to clinical trials has been awarded the first of what researchers hope will be several grants from the National Cancer Institute.
-
Full Story
GSIP: Summer of 2024 Reflections
 8/15/2024
We hope our students’ individual notes and the photos within this booklet demonstrate the variety of experiences working in Gates Institutemembers’ labs. Over 11 weeks, these talented students developed their projects and participated in an array of seminars and events designed to expose them to high-impact opportunities and career paths. They excelled in their laboratory placements and developed friendships and connections with their mentors, fellow lab and Gates Institute staff and our speakers. Ultimately, their contributions advanced medical research on the Anschutz Medical Campus – a fertile environment we hope they will return to
8/15/2024
We hope our students’ individual notes and the photos within this booklet demonstrate the variety of experiences working in Gates Institutemembers’ labs. Over 11 weeks, these talented students developed their projects and participated in an array of seminars and events designed to expose them to high-impact opportunities and career paths. They excelled in their laboratory placements and developed friendships and connections with their mentors, fellow lab and Gates Institute staff and our speakers. Ultimately, their contributions advanced medical research on the Anschutz Medical Campus – a fertile environment we hope they will return to
-
Full Story
Better. Stronger. Faster. Scientists Rebuild Cancer-Killing Cells
 8/6/2024
When Eduardo Davila, PhD, talks about his work to leverage a patient’s immune system to fight cancer, it reminds him of a TV show he watched as a child: “The Six Million Dollar Man.” The show’s main character, after a devastating accident, is rebuilt into a better and stronger version of himself.
8/6/2024
When Eduardo Davila, PhD, talks about his work to leverage a patient’s immune system to fight cancer, it reminds him of a TV show he watched as a child: “The Six Million Dollar Man.” The show’s main character, after a devastating accident, is rebuilt into a better and stronger version of himself.
-
Full Story
Gates Biomanufacturing Facility Prepares for Annual Shutdown
 7/26/2024
The Gates Biomanufacturing Facility (GBF) is gearing up for what has become a summertime tradition. It has nothing to do with activities associated with summer; the purpose is to keep the operation running smoothly year-round.
7/26/2024
The Gates Biomanufacturing Facility (GBF) is gearing up for what has become a summertime tradition. It has nothing to do with activities associated with summer; the purpose is to keep the operation running smoothly year-round.
-
Full Story
Gates Summer Internship Program Celebrates its First Decade
 7/12/2024
A crowd of about 100 turned out to celebrate the 10th anniversary of the Gates Summer Internship Program (GSIP) on June 28 at the Anschutz Health Sciences Building, marking a milestone for a program designed to help train the next generation of regenerative medicine researchers. Attendees included program supporters and friends, program alumni and mentors, 21 members of the Class of 2024, and Gates Institute staff. Gates Institute COO Laura Borgelt, PharmD, MBA, kicked off the festivities with her remarks, noting that GSIP was launched at a pivotal time in the field of regenerative
7/12/2024
A crowd of about 100 turned out to celebrate the 10th anniversary of the Gates Summer Internship Program (GSIP) on June 28 at the Anschutz Health Sciences Building, marking a milestone for a program designed to help train the next generation of regenerative medicine researchers. Attendees included program supporters and friends, program alumni and mentors, 21 members of the Class of 2024, and Gates Institute staff. Gates Institute COO Laura Borgelt, PharmD, MBA, kicked off the festivities with her remarks, noting that GSIP was launched at a pivotal time in the field of regenerative
-
Full Story
Charles River Laboratories Announces Strategic Lentiviral Vector Manufacturing Collaboration with Gates Institute
 6/25/2024
Rockville, MD. – June 25, 2024 – Charles River Laboratories International, Inc. (NYSE: CRL) and the Gates Institute at the University of Colorado Anschutz Medical Campus today announced a lentiviral vector contract development and manufacturing organization (CDMO) agreement. Gates Institute will leverage Charles River’s premier cell and gene therapy CDMO expertise to develop Good Manufacturing Practice (GMP)-grade lentiviral vectors (LVVs) for use in novel chimeric antigen receptor (CAR) T-cell therapies for hematological cancers.
6/25/2024
Rockville, MD. – June 25, 2024 – Charles River Laboratories International, Inc. (NYSE: CRL) and the Gates Institute at the University of Colorado Anschutz Medical Campus today announced a lentiviral vector contract development and manufacturing organization (CDMO) agreement. Gates Institute will leverage Charles River’s premier cell and gene therapy CDMO expertise to develop Good Manufacturing Practice (GMP)-grade lentiviral vectors (LVVs) for use in novel chimeric antigen receptor (CAR) T-cell therapies for hematological cancers.
-
Full Story
Paving the Way Toward Transplantation: NIH Awards Support Ophthalmology Retinal Research
 6/25/2024
Researchers in the Department of Ophthalmology at the University of Colorado School of Medicine have received nearly $4 million in R01 grants from the National Institutes of Health (NIH) to move forward in their work at CellSight, the Ocular Stem Cell and Regeneration Research Program based at the Sue Anschutz-Rodgers Eye Center.
6/25/2024
Researchers in the Department of Ophthalmology at the University of Colorado School of Medicine have received nearly $4 million in R01 grants from the National Institutes of Health (NIH) to move forward in their work at CellSight, the Ocular Stem Cell and Regeneration Research Program based at the Sue Anschutz-Rodgers Eye Center.
-
Full Story
Getting to Know Maureen O’Brien, MD, MS
 6/20/2024
Maureen O’Brien, MD, MS, recently joined the faculty of University of Colorado Anschutz School of Medicine as a visiting professor in the Department of Pediatrics Section of Hematology/Oncology and Bone Marrow Transplantation. With support from Gates Institute and in collaboration with colleagues leading the adult leukemia programs, O’Brien will develop and help lead a campuswide high-risk leukemia program. This program will facilitate the implementation of early-phase clinical trials for pediatric and adult leukemia, including cellular therapies developed with the Gates Institute.
6/20/2024
Maureen O’Brien, MD, MS, recently joined the faculty of University of Colorado Anschutz School of Medicine as a visiting professor in the Department of Pediatrics Section of Hematology/Oncology and Bone Marrow Transplantation. With support from Gates Institute and in collaboration with colleagues leading the adult leukemia programs, O’Brien will develop and help lead a campuswide high-risk leukemia program. This program will facilitate the implementation of early-phase clinical trials for pediatric and adult leukemia, including cellular therapies developed with the Gates Institute.
-
Full Story
Navigating Operational Complexities in Phase I GMP Cell Therapy Manufacturing: A Compliance Perspective
 6/11/2024
In the rapidly evolving field of cell therapy, the transition from research to clinical application is filled with challenges, particularly within Good Manufacturing Practice (GMP) production during first-in-human, early-phase clinical trials. These trials set the groundwork for both safety (primarily) and efficacy (secondarily) as therapies advance toward commercialization. Understanding the operational intricacies involved in manufacturing cell therapy products for these trials is crucial for stakeholders, including sponsors, innovators, investors, and regulatory compliance professionals.
6/11/2024
In the rapidly evolving field of cell therapy, the transition from research to clinical application is filled with challenges, particularly within Good Manufacturing Practice (GMP) production during first-in-human, early-phase clinical trials. These trials set the groundwork for both safety (primarily) and efficacy (secondarily) as therapies advance toward commercialization. Understanding the operational intricacies involved in manufacturing cell therapy products for these trials is crucial for stakeholders, including sponsors, innovators, investors, and regulatory compliance professionals.
-
Full Story
Gates Institute Taps Leading Biotech Experts for New Advisory Board
 6/10/2024
Five individuals with biotechnology expertise have been recruited by Gates Institute to form a new scientific advisory board. They will provide strategic and scientific input to guide specific cell and gene therapy programs, with a focus on increasing patient impact, prioritizing near- and long-term goals, and exploring new platforms, said Gates Institute Executive Director Terry Fry, MD.
6/10/2024
Five individuals with biotechnology expertise have been recruited by Gates Institute to form a new scientific advisory board. They will provide strategic and scientific input to guide specific cell and gene therapy programs, with a focus on increasing patient impact, prioritizing near- and long-term goals, and exploring new platforms, said Gates Institute Executive Director Terry Fry, MD.
-
Full Story
Gates Summer Internship Program Marks 10 Years of Mentorships and Discovery
 6/4/2024
When the Gates Summer Internship Program (GSIP) launched in 2015, the field of regenerative medicine was at a pivotal moment, with recent developments in cell and gene therapy being heralded by the scientific community.
6/4/2024
When the Gates Summer Internship Program (GSIP) launched in 2015, the field of regenerative medicine was at a pivotal moment, with recent developments in cell and gene therapy being heralded by the scientific community.
-
Full Story
Fulfilling a Dream: Ophthalmology Researcher Works to Restore People’s Vision
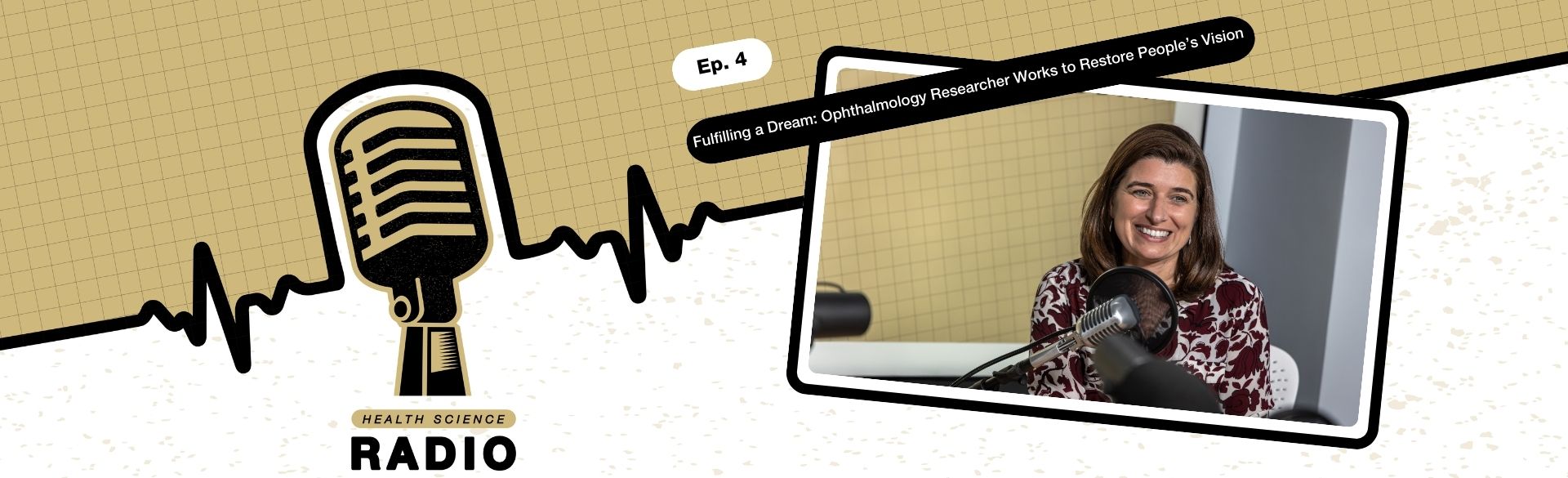 5/6/2024
Having a close friend who was blind as a teenager gave Valeria Canto-Soler, PhD, a clear vision for her future. She wanted to help people with vision problems recover their sight.
5/6/2024
Having a close friend who was blind as a teenager gave Valeria Canto-Soler, PhD, a clear vision for her future. She wanted to help people with vision problems recover their sight.
-
Full Story
Multi-Campus Effort Aims to Regenerate Arthritic Joints
 4/19/2024
Osteoarthritis, a painful degenerative disease that affects 32.5 million Americans, slowly degrades buffering cartilage until joints grind together bone-on-bone. With no existing effective regenerative therapy, treatments are limited to anti-inflammatory injections and, ultimately, expensive joint replacement surgery.
4/19/2024
Osteoarthritis, a painful degenerative disease that affects 32.5 million Americans, slowly degrades buffering cartilage until joints grind together bone-on-bone. With no existing effective regenerative therapy, treatments are limited to anti-inflammatory injections and, ultimately, expensive joint replacement surgery.
-
Full Story
From Organ Donor to Clinical Research Expert
 4/3/2024
From an early age, Cheri Adams, program director of regulatory strategy at the Gates Institute, knew she would pursue a career in medicine. But it was her experience as an organ donor that led her to a career in clinical research.
4/3/2024
From an early age, Cheri Adams, program director of regulatory strategy at the Gates Institute, knew she would pursue a career in medicine. But it was her experience as an organ donor that led her to a career in clinical research.
-
Full Story
Joints That Could Heal Themselves? Researchers Could Get There in Five Years
 3/26/2024
Imagine a day when joints could heal themselves.
3/26/2024
Imagine a day when joints could heal themselves.
-
Full Story
Nephrologist Aims to Explore CAR T Therapy for Solid Tumors
 3/18/2024
Chimeric antigen receptor (CAR) T-cell therapy has shown great promise in the treatment of certain leukemias and lymphoma, but results in solid tumors have not been as impressive.
3/18/2024
Chimeric antigen receptor (CAR) T-cell therapy has shown great promise in the treatment of certain leukemias and lymphoma, but results in solid tumors have not been as impressive.
-
Full Story
CU studying use of patients’ own reprogrammed cells to attack cancer as alternative to more chemo
 3/14/2024
A study at University of Colorado’s Gates Institute on the Anschutz Medical Campus is looking at CAR-T in adult patients with acute lymphocytic leukemia, a cancer of the blood and bone marrow, whose first round of chemotherapy either failed or gave a disappointing response that suggests it won’t work for long, executive director Dr. Terry Fry said. (The institute is named for rubber manufacturer Charles C. Gates.)
3/14/2024
A study at University of Colorado’s Gates Institute on the Anschutz Medical Campus is looking at CAR-T in adult patients with acute lymphocytic leukemia, a cancer of the blood and bone marrow, whose first round of chemotherapy either failed or gave a disappointing response that suggests it won’t work for long, executive director Dr. Terry Fry said. (The institute is named for rubber manufacturer Charles C. Gates.)
-
Full Story
Project to Rev Up Cancer-Fighting Cells Wins Anschutz Acceleration Initiative Grant
 2/2/2024
A project to develop a way to boost the effectiveness of cellular cancer therapies, led by the University of Colorado Cancer Center’s Associate Director of Cancer Research Training and Education Coordination, Eduardo Davila, PhD, is one of nine research endeavors by CU School of Medicine faculty members to be awarded major funding from the Anschutz Acceleration Initiative.
2/2/2024
A project to develop a way to boost the effectiveness of cellular cancer therapies, led by the University of Colorado Cancer Center’s Associate Director of Cancer Research Training and Education Coordination, Eduardo Davila, PhD, is one of nine research endeavors by CU School of Medicine faculty members to be awarded major funding from the Anschutz Acceleration Initiative.
-
Full Story
Gates Grubstake Fund Awards over $1.4 Million to CU Anschutz Researchers
 1/8/2024
Gates Institute recently announced four recipients of the 2023 Gates Grubstake Fund. These awards are designed to support investigators who are researching and developing regenerative medicine-related technologies. The awards of up to $350,000 are made annually in a process administered in collaboration with CU Innovations. The fund made awards totaling over $1.4 million to University of Colorado Anschutz researchers in 2023.
1/8/2024
Gates Institute recently announced four recipients of the 2023 Gates Grubstake Fund. These awards are designed to support investigators who are researching and developing regenerative medicine-related technologies. The awards of up to $350,000 are made annually in a process administered in collaboration with CU Innovations. The fund made awards totaling over $1.4 million to University of Colorado Anschutz researchers in 2023.
-
Full Story
Gates Institute Names Navin Pinto as Medical Lead
 1/8/2024
Navin Pinto, MD, a professor in the Department of Pediatrics at the University of Colorado School of Medicine on the CU Anschutz Medical Campus, has been named medical lead at Gates Institute. In this role, he’ll work in partnership with the Investigational New Drug and Device (IND/IDE) Office to oversee Gates Institute-supported clinical trials, providing expertise drawn from his extensive experience with chimeric antigen receptor (CAR) T-cell therapy.
1/8/2024
Navin Pinto, MD, a professor in the Department of Pediatrics at the University of Colorado School of Medicine on the CU Anschutz Medical Campus, has been named medical lead at Gates Institute. In this role, he’ll work in partnership with the Investigational New Drug and Device (IND/IDE) Office to oversee Gates Institute-supported clinical trials, providing expertise drawn from his extensive experience with chimeric antigen receptor (CAR) T-cell therapy.
-
Full Story
The Latest News About CAR T-Cell Therapy
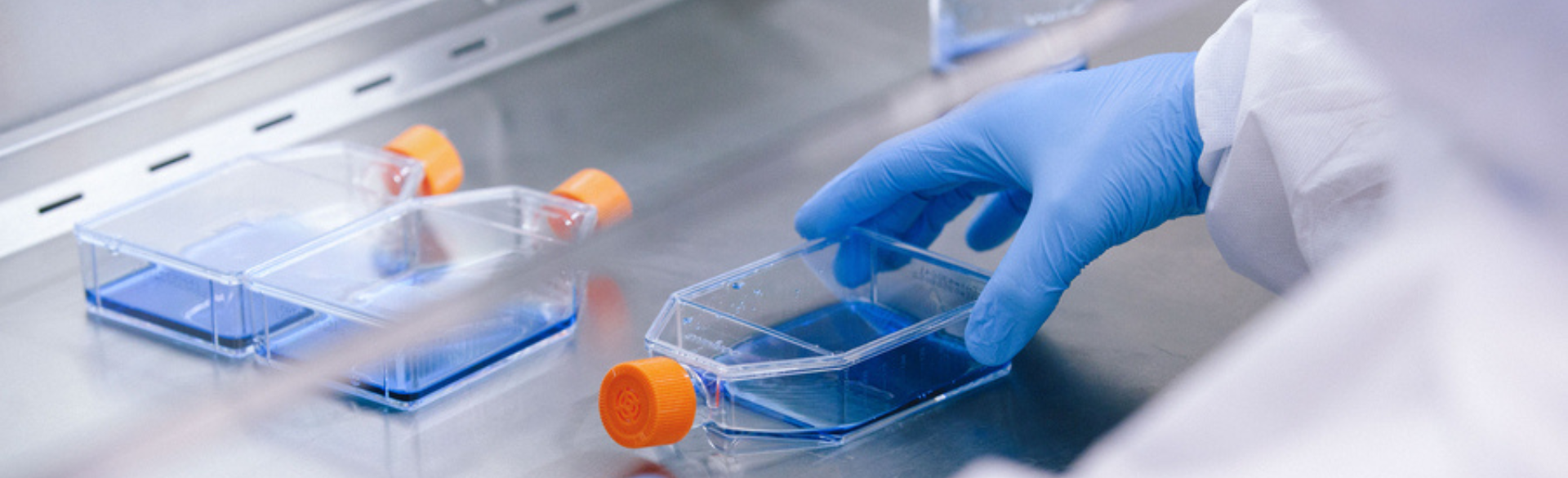 1/3/2024
As word of the effectiveness of chimeric antigen receptor, or CAR T-cell therapy, for blood cancer continues to spread, excitement is growing about the new treatment and the possibilities it offers for patients with blood cancers and other types of cancer.
1/3/2024
As word of the effectiveness of chimeric antigen receptor, or CAR T-cell therapy, for blood cancer continues to spread, excitement is growing about the new treatment and the possibilities it offers for patients with blood cancers and other types of cancer.
-
Full Story
Gates Institute’s Top Stories of 2023
 12/21/2023
It’s been a banner year for the Gates Institute, which celebrated its launch in May 2023, consolidating the Gates Biomanufacturing Facility with the administrative team of the former Gates Center for Regenerative Medicine as well as the regulatory expertise of the Clinical Investigation and Regulatory Sciences (CELLS) team under one umbrella. The year has seen the launch of a new chimeric antigen receptor (CAR) T-cell clinical trial and continued enrollment of three existing CAR T-cell trials. The Gates Institute newsroom detailed these activities, as well as other areas of cell and gene therapy research
12/21/2023
It’s been a banner year for the Gates Institute, which celebrated its launch in May 2023, consolidating the Gates Biomanufacturing Facility with the administrative team of the former Gates Center for Regenerative Medicine as well as the regulatory expertise of the Clinical Investigation and Regulatory Sciences (CELLS) team under one umbrella. The year has seen the launch of a new chimeric antigen receptor (CAR) T-cell clinical trial and continued enrollment of three existing CAR T-cell trials. The Gates Institute newsroom detailed these activities, as well as other areas of cell and gene therapy research
-
Full Story
CU Anschutz Harnesses Technology and Innovation to Speed Drug Discovery
 12/15/2023
In the best of cases, taking a new drug from lab to clinic takes about six to eight years, a vast improvement over the roughly 20-year timeline decades ago. Drug development pace and efficiency are leaping even farther ahead, courtesy of quantum computing, artificial intelligence algorithms and 3D tissue printers, especially at the University of Colorado Anschutz Medical Campus.
12/15/2023
In the best of cases, taking a new drug from lab to clinic takes about six to eight years, a vast improvement over the roughly 20-year timeline decades ago. Drug development pace and efficiency are leaping even farther ahead, courtesy of quantum computing, artificial intelligence algorithms and 3D tissue printers, especially at the University of Colorado Anschutz Medical Campus.
-
Full Story
Gates Biomanufacturing Facility Deploys CAR T-Cell Process Improvement
 12/15/2023
Since receiving FDA approval in 2017, chimeric antigen receptor (CAR) T-cell therapy has shown remarkable success in treating patients with certain blood cancers such as lymphoma and leukemia. A complex biomanufacturing process is required to produce the therapy, which is performed for each individual patient. The process begins with apheresis, generating a leukopak containing the patient’s white blood cells. From there, T cells are isolated, genetically engineered to create CAR proteins that enable T cells to attack cancer cells, and multiplied in a laboratory. They are then reintroduced to the patient.
12/15/2023
Since receiving FDA approval in 2017, chimeric antigen receptor (CAR) T-cell therapy has shown remarkable success in treating patients with certain blood cancers such as lymphoma and leukemia. A complex biomanufacturing process is required to produce the therapy, which is performed for each individual patient. The process begins with apheresis, generating a leukopak containing the patient’s white blood cells. From there, T cells are isolated, genetically engineered to create CAR proteins that enable T cells to attack cancer cells, and multiplied in a laboratory. They are then reintroduced to the patient.
-
Full Story
CellSight Contributes Light-Sensitive Retinal Organoids and RPE Cells to New AMD Study
 12/6/2023
A partnership between ophthalmology researchers at the University of Colorado School of Medicine and Johns Hopkins University expands the understanding of how oxidative stress contributes to the development of choroidal neovascularization (CNV) in patients with age-related macular degeneration (AMD).
12/6/2023
A partnership between ophthalmology researchers at the University of Colorado School of Medicine and Johns Hopkins University expands the understanding of how oxidative stress contributes to the development of choroidal neovascularization (CNV) in patients with age-related macular degeneration (AMD).
-
Full Story
Patch May Successfully Treat Congenital Heart Defects
 11/27/2023
Using laboratory engineered tissue, scientists at the University of Colorado Anschutz Medical Campus have created a full thickness, biodegradable patch that holds the promise of correcting congenital heart defects in infants, limiting invasive surgeries and outlasting current patches.
11/27/2023
Using laboratory engineered tissue, scientists at the University of Colorado Anschutz Medical Campus have created a full thickness, biodegradable patch that holds the promise of correcting congenital heart defects in infants, limiting invasive surgeries and outlasting current patches.
-
Full Story
Trial Launched to Test CAR T-Cell Therapy in Dogs Diagnosed With Solid Tumors
 11/7/2023
Dogs are like humans in many ways, sharing similar physiology as well as biological needs. Our four-legged friends are also vulnerable to some of the same diseases that we face, making the intersection of human and animal medicine an intriguing subject for study.
11/7/2023
Dogs are like humans in many ways, sharing similar physiology as well as biological needs. Our four-legged friends are also vulnerable to some of the same diseases that we face, making the intersection of human and animal medicine an intriguing subject for study.
-
Full Story
Gene Therapy Protocol Could Offer New Treatment for Skin-blistering Diseases
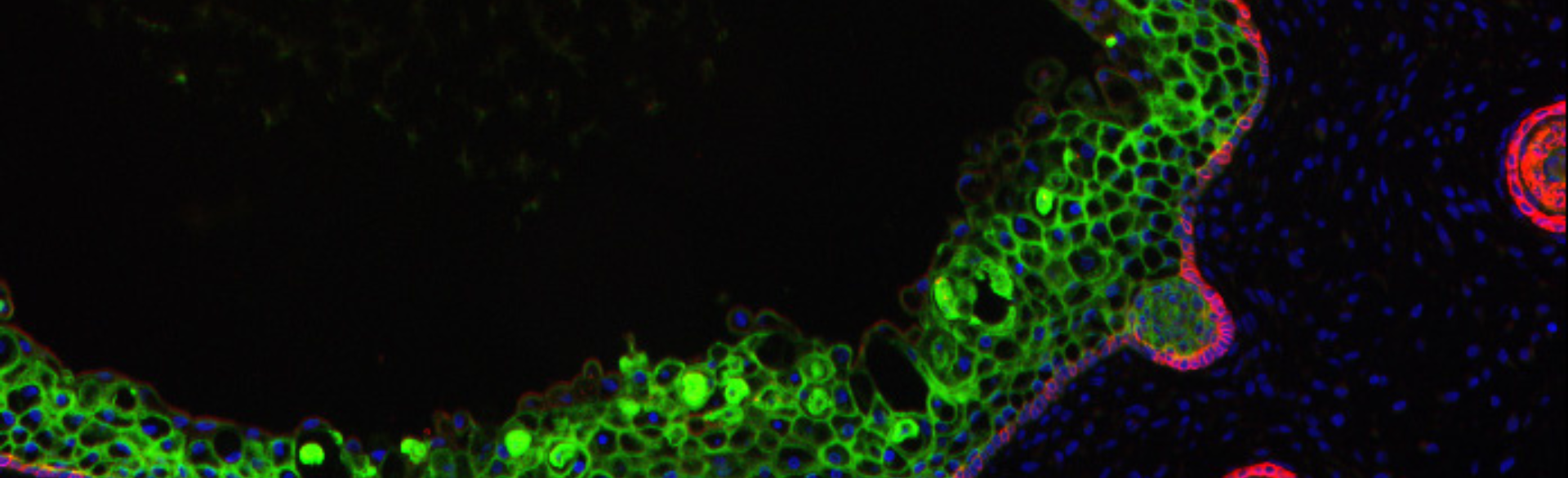 10/27/2023
Epidermolysis bullosa (EB) is a skin-blistering disease that can be devastating for those with severe forms of the condition. Research is underway to grow genetically corrected skin by Gates Institute investigators Ganna Bilousova, PhD, and Igor Kogut, PhD, associate professors of dermatology at University of Colorado School of Medicine, in collaboration with Dennis Roop, PhD, professor of dermatology and associate director of the Gates Institute. Their work could lead to effective therapies for EB as well as other diseases.
10/27/2023
Epidermolysis bullosa (EB) is a skin-blistering disease that can be devastating for those with severe forms of the condition. Research is underway to grow genetically corrected skin by Gates Institute investigators Ganna Bilousova, PhD, and Igor Kogut, PhD, associate professors of dermatology at University of Colorado School of Medicine, in collaboration with Dennis Roop, PhD, professor of dermatology and associate director of the Gates Institute. Their work could lead to effective therapies for EB as well as other diseases.
-
Full Story
Technology Transfer: The Key to Moving Scientific Discoveries Forward
 9/26/2023
At the surface, technology transfer is exactly what it sounds like; the transfer of technology from one entity to another. When working with your friendly neighborhood cGMP facility (the Gates Biomanufacturing Facility, or GBF), that simple phrase, technology transfer, should take on a whole new meaning.
9/26/2023
At the surface, technology transfer is exactly what it sounds like; the transfer of technology from one entity to another. When working with your friendly neighborhood cGMP facility (the Gates Biomanufacturing Facility, or GBF), that simple phrase, technology transfer, should take on a whole new meaning.
-
Full Story
Behind the Scenes of a CAR T-Cell Trial
 9/15/2023
Blood cancers – leukemia, lymphoma and myeloma -- are the third-leading cause of cancer deaths in the United States, with more than one-third of patients succumbing to their disease within five years of diagnosis. In 2017, the Food and Drug Administration approved the use of chimeric antigen receptor (CAR) T-cell therapy for lymphoma and certain leukemias in patients for whom other treatments had failed. Research has shown that about 85% of such patients achieve remission, but about 60% of these eventually relapse. Gates Institute at the University of Colorado Anschutz Medical Campus is on
9/15/2023
Blood cancers – leukemia, lymphoma and myeloma -- are the third-leading cause of cancer deaths in the United States, with more than one-third of patients succumbing to their disease within five years of diagnosis. In 2017, the Food and Drug Administration approved the use of chimeric antigen receptor (CAR) T-cell therapy for lymphoma and certain leukemias in patients for whom other treatments had failed. Research has shown that about 85% of such patients achieve remission, but about 60% of these eventually relapse. Gates Institute at the University of Colorado Anschutz Medical Campus is on
-
Full Story
From Childhood Fascination With Red Blood Cells to Life-Changing Research
 9/11/2023
When he was 4 years old, Angelo D’Alessandro clearly recalls a cartoon book about the peripatetic nature of red blood cells. Their adventures traveling through the body, visiting the brain, kidneys, lungs, liver, et al., mesmerized D’Alessandro in his native Italy.
9/11/2023
When he was 4 years old, Angelo D’Alessandro clearly recalls a cartoon book about the peripatetic nature of red blood cells. Their adventures traveling through the body, visiting the brain, kidneys, lungs, liver, et al., mesmerized D’Alessandro in his native Italy.
-
Full Story
GSIP: Summer of 2023 Reflections
 9/1/2023
We hope our students’ individual notes and the photos within this booklet demonstrate the variety of experiences working in Gates Institutemembers’ labs. Over 11 weeks, these talented students developed their projects and participated in an array of seminars and events designed to expose them to high-impact opportunities and career paths. They excelled in their laboratory placements and developed friendships and connections with their mentors, fellow lab and Gates Institute staff and our speakers. Ultimately, their contributions advanced medical research on the Anschutz Medical Campus – a fertile environment we hope they will return to
9/1/2023
We hope our students’ individual notes and the photos within this booklet demonstrate the variety of experiences working in Gates Institutemembers’ labs. Over 11 weeks, these talented students developed their projects and participated in an array of seminars and events designed to expose them to high-impact opportunities and career paths. They excelled in their laboratory placements and developed friendships and connections with their mentors, fellow lab and Gates Institute staff and our speakers. Ultimately, their contributions advanced medical research on the Anschutz Medical Campus – a fertile environment we hope they will return to
-
Full Story
Gates Summer Internship Program Celebrates the Class of 2023
 8/16/2023
This summer marked the ninth year of the Gates Summer Internship Program (GSIP), which has become a hallmark of Gates Institute’s collective efforts to inspire outstanding college undergraduates to pursue careers in stem cell biology and regenerative medicine. GSIP combines lab work, seminars focused on science and medicine, workshops on ethics and professional development, as well as outside activities that allow the interns to experience Colorado beyond the University of Colorado Anschutz Medical Campus.
8/16/2023
This summer marked the ninth year of the Gates Summer Internship Program (GSIP), which has become a hallmark of Gates Institute’s collective efforts to inspire outstanding college undergraduates to pursue careers in stem cell biology and regenerative medicine. GSIP combines lab work, seminars focused on science and medicine, workshops on ethics and professional development, as well as outside activities that allow the interns to experience Colorado beyond the University of Colorado Anschutz Medical Campus.
-
Full Story
Student With Rare Genetic Condition Searches for a Cure at Gates Institute
 7/28/2023
Few college seniors can say they are working in a world-renowned lab to develop therapies for a genetic disorder that plagues 1 in 5,000 people worldwide.
7/28/2023
Few college seniors can say they are working in a world-renowned lab to develop therapies for a genetic disorder that plagues 1 in 5,000 people worldwide.


