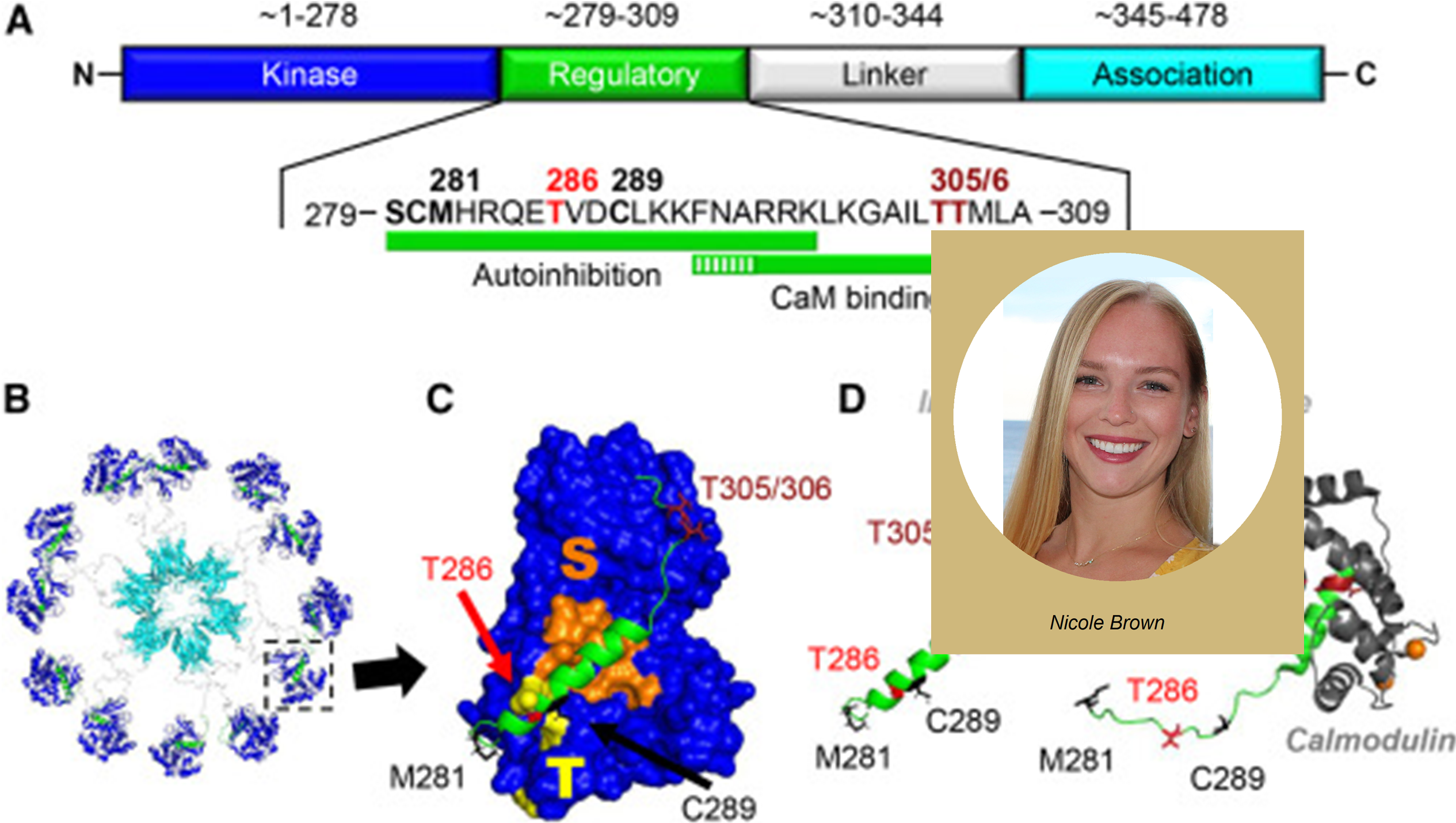
Department of Pharmacology
School of Medicine | Anschutz Medical Campus
Pharmacology Retreat 2023
Keystone Lodge & Spa
Anschutz Medical Campus
Pharmacology Staff & Students
Fall 2022
Welcome to the Department of Pharmacology
Located just outside of the beautiful city of Denver, Colorado, and in close proximity to the Rocky Mountains, our department is actively engaged in uncovering fundamental mechanisms of biological systems, so that they can be targeted and manipulated in a therapeutic context to treat or prevent disease.
- About Us
- Research Areas
- We consistently rank in the top tier for NIH funding for Pharmacology Departments.
- The Pharmacology Graduate Program is one of the longest standing NIH funded programs in the country.
| Other Graduate Programs affiliated with Pharmacology Faculty | |||
| Cancer Biology | Molecular Biology | Neuroscience | Structural Biology |
| Computational Bioscience | Biomedical Sciences | Medical Scientist Training Program | |
About Pharmacology
Department News
Pharmacology Fall 2023 Newsletter
Research Areas