At the Ludeman Family Center for Women's Health Research, we believe that education, research and community outreach has the power to fundamentally improve lives for women—and for everyone.
![]()
Research
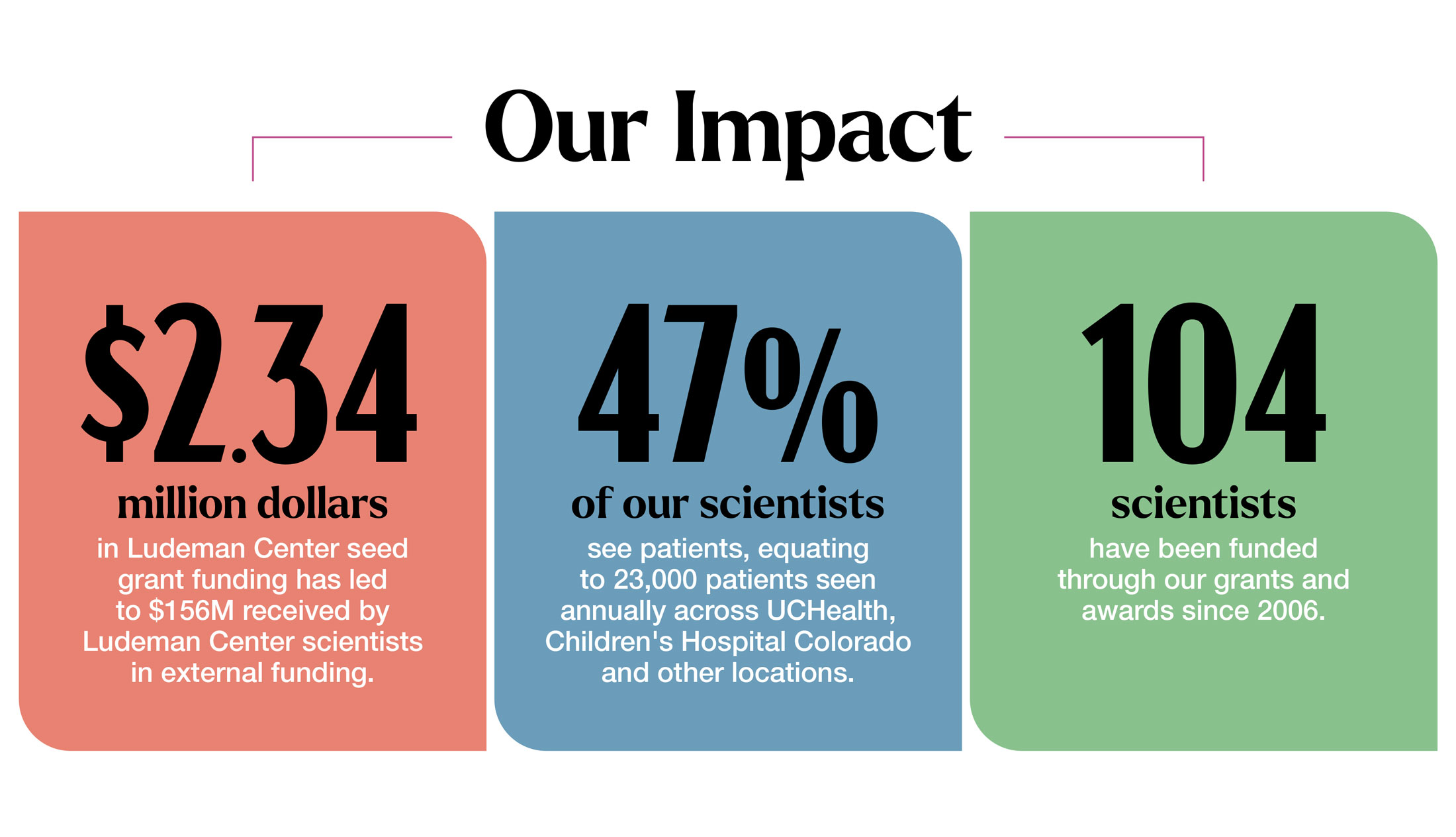
The Ludeman Center fuels research in women’s health and sex and gender differences to improve the health of women, their families and communities. Our grants are powered by generous donors and support our scientists to go on to receive national funding, bringing their lifesaving discoveries from the lab to the public.
Mentorship
Through mentorship and funding, we support the next generation of scientists whose cutting-edge research focuses on issues that pose a significant threat to women’s health. This includes dedicated research to cardiovascular disease, diabetes and the intersection of mental health and physical health.
Community Outreach
Our virtual and in-person educational programs connect the public to leading researchers and experts in women's health research. The Let's Talk series focuses on trending health topics to empower you and your healthcare providers to make the most informed decisions while our annual Girls Career Day is dedicated to supporting high school-aged girls to explore careers in healthcare and research.
Our Work in Action
Funding Opportunities

Through our grant programs and dedicated mentorship, we support scientists studying women’s health and sex and gender differences research.
Upcoming Events

Learn, engage, participate, and support women's health issues across the lifespan through community events.
Women's Health News

Sharing groundbreaking research, innovative studies, important health tips and stories of how research is improving lives.

Stay Connected to Our Work
![]()